- Institute
- Research topics
- Organization
- Platforms
- Services
- Europe/International
- Science outreach
- Agenda
- Directory
- Access

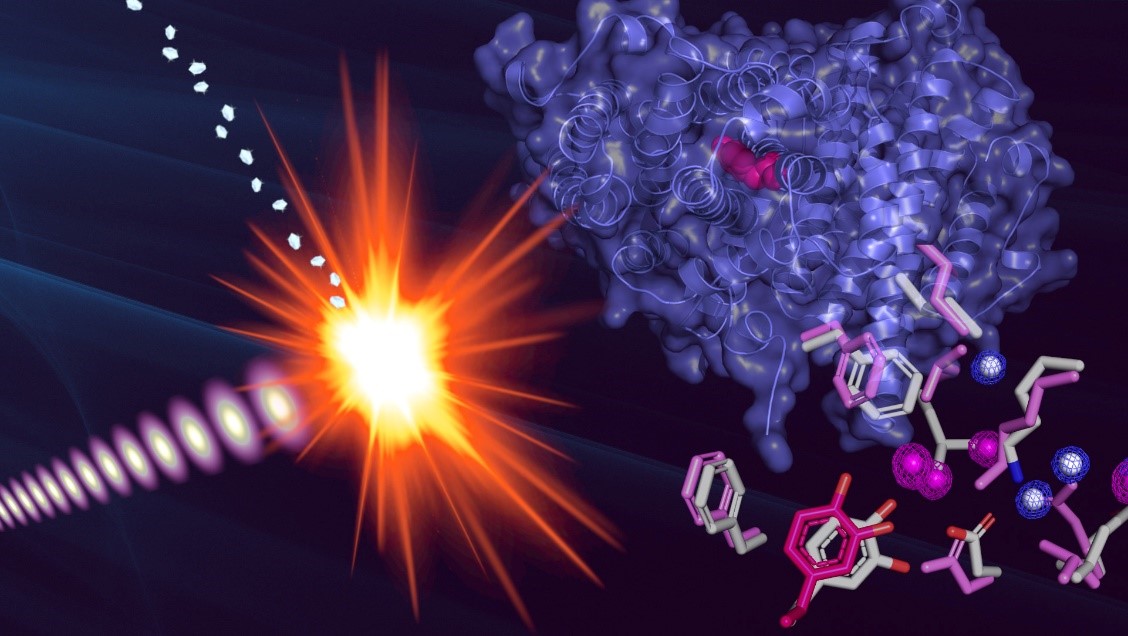
Enzymes are involved in essential biological processes, but their fragility is an obstacle to obtaining their intact structures using conventional structural biology methods.
Hugo Lebrette (LMGM-CBI) and his colleagues were able to observe for the first time the atomic structure of the radical state, an extremely reactive state, of an enzyme, the mycoplasma ribonucleotide reductase, using serial crystallography coupled to an ultrafast laser.
Hugo Lebrette and his colleagues met this challenge using the concept of "diffraction before destruction", by illuminating a stream of protein crystals with an ultrafast laser where each pulse lasts a few femtoseconds.
This result, which provides an unprecedented understanding of the structure of an enzyme, opens the way to potential therapeutic innovations.
© Martin Högbom et Hugo Lebrette
Figure : Structure de la sous-unité R2 de la ribonucléotide réductase de mycoplasme obtenue par cristallographie femtoseconde en série par laser à rayons X à électrons libres. La superposition des acides aminés entre les deux états, radicalaire (rose) et non-radicalaire (gris), montre les changements conformationnels induits par l’acquisition du radical.
 Read more :
Read more :
 Contact : Hugo Lebrette
Contact : Hugo Lebrette